The terms opiate and opioid are often used interchangeably, different authors define what is considered an opiate or an opioid with little consistency. The suffix "-iod" means like, hence opioids act like opiates. In general opiate refers to alkaloids found in the opium poppy that intereact with the body's endorphin receptors. Opioids are molecules that interact with the same receptors but are either fully synthetic (ie fentanyl), derived from the alkaloids found in opium and thus semi-synthetic (ie buprenorphine), and molecules from other plants that are distinct from morphine but nonetheless do interact with the opioid receptors (eg kratom). For the purposes of this blog, I define opiates are any molecules with only minor derivations on morphine. The following is a discussion of five common pharmaceutical narcotics and how they relate to the prototypical opiate, morphine. I consider codeine, hydromorphone, oxymorphone, hydrocodone and oxycodone all to be opiates. The most common changes to the morphine molecule involves:
1. Changing substituents at carbons 3 and 6. In morphine these are alcohol (-OH) groups.
2. Reduction of the double bond between carbons 7 and 8.
3. Addition of an alcohol (-OH). group at carbon 14.
4. Addition or changes to the group coming off the nitrogen, carbon #17.
Figure 1. Morphine with the carbon atoms numbered. Morphine is the primary alkaloid in opium.
 Codeine is also found naturally in opium, and in (slightly) more enlightened countries is sold over the counter, though never without added acetaminophen (Tylenol) or acetylsalicylic acid (aspirin). Codeine is identical to morphine but has a methyl group attached to the oxygen on carbon #3. A carbon-oxygen-carbon grouping is known as an ether, thus codeine is 3-methyl ether morphine. This dramatically reduces the activity of codeine to only 10% of morphine.
Codeine is also found naturally in opium, and in (slightly) more enlightened countries is sold over the counter, though never without added acetaminophen (Tylenol) or acetylsalicylic acid (aspirin). Codeine is identical to morphine but has a methyl group attached to the oxygen on carbon #3. A carbon-oxygen-carbon grouping is known as an ether, thus codeine is 3-methyl ether morphine. This dramatically reduces the activity of codeine to only 10% of morphine. Hydromorphone has two changes to the morphine molecule which increases its relative potency. The OH group at position 3 in morphine has the hydrogen removed, the oxygen is now double bonded to carbon 6. A carbon-oxygen double bond is known as a ketone ("key-tone"), thus the "-one" at the end of hydromorphone. The double bond between carbons 7 and 8 has been reduced to a single bond, by adding two hydrogen atoms (H not shown). This should make the molecule "dihydro-morphin-one," due to the addition of two hydrogen molecules (dihydro) and the oxidation of the OH group at carbon 6 to a ketone (morph-INE to morph-ONE). However the name is derived not from the double bond between carbon's 7 and 8, but for the atom bonded to carbon 14. In this case hydromorphone retains the same configuration as morphine, a single H at carbon 14.
Hydromorphone has two changes to the morphine molecule which increases its relative potency. The OH group at position 3 in morphine has the hydrogen removed, the oxygen is now double bonded to carbon 6. A carbon-oxygen double bond is known as a ketone ("key-tone"), thus the "-one" at the end of hydromorphone. The double bond between carbons 7 and 8 has been reduced to a single bond, by adding two hydrogen atoms (H not shown). This should make the molecule "dihydro-morphin-one," due to the addition of two hydrogen molecules (dihydro) and the oxidation of the OH group at carbon 6 to a ketone (morph-INE to morph-ONE). However the name is derived not from the double bond between carbon's 7 and 8, but for the atom bonded to carbon 14. In this case hydromorphone retains the same configuration as morphine, a single H at carbon 14.  Oxymorphone has the same two changes to the morphine molecule as hydromorphone, but also has an OH group attached to carbon 14 in place of the hydrogen in morphine. This increases the potency and is the reason the name is OXY-morph-ONE. The oxy prefix refers to the OH on carbon 14, and the one suffix refers to the change at carbon 6.
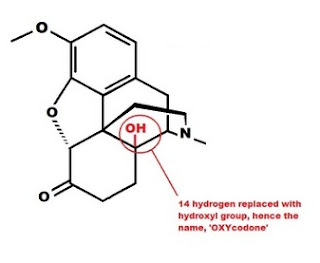
Oxymorphone has the same two changes to the morphine molecule as hydromorphone, but also has an OH group attached to carbon 14 in place of the hydrogen in morphine. This increases the potency and is the reason the name is OXY-morph-ONE. The oxy prefix refers to the OH on carbon 14, and the one suffix refers to the change at carbon 6.
Both oxycodone and hydrocodone involve the same changes to the morphine molecule as oxymorphone and hydromorphone, but include the methyl (-CH3) group attached to the 3rd carbon just like codeine.

The changes to the morphine structure can be summarized as follows:
A. Addition of a methyl group to the oxygen on carbon 3. Creates a methyl-3-ether linkage. Reduces potency.
B. Alcohol group (-OH) on carbon 6 oxidized to a double bonded ketone (=O). Increases potency.
C. Hydrogenation (two H atoms are added) of double bond between carbons 7 and 8. Increases potency.
D. Substitution of an alcohol group (-OH) for the hydrogen at carbon 14. Increases potency.
Opiate Changes to Morphine Brand Names
Morphine - MS Contin
Codeine A Paramol, Tylenol 3
Hydromorphone B, C Dilaudid, Palladone
Oxymorphone B, C, D Opana, Numorphan, Numorphone
Hydrocodone A, B, C Vicodin, Lortab
Oxycodone A, B, C, D Oxycontin, Percocet, (More here)
 Oxycodone is an example of all four changes to the basic morphine structure. A decreases the potency, while B, C and D increase the potency. The net result is a molecule slightly more potent than morphine, though far less potent than oxymorphone.
Oxycodone is an example of all four changes to the basic morphine structure. A decreases the potency, while B, C and D increase the potency. The net result is a molecule slightly more potent than morphine, though far less potent than oxymorphone.  Narcotic antagonists are made by using the oxymorphone structure with modified substituents on the nitrogen. The groups off the nitrogen have major effects on the pharmacological activity. Both naloxone and naltrexone are used to reverse opioid overdoses and have no intrinsic opioid activity of their own.
Narcotic antagonists are made by using the oxymorphone structure with modified substituents on the nitrogen. The groups off the nitrogen have major effects on the pharmacological activity. Both naloxone and naltrexone are used to reverse opioid overdoses and have no intrinsic opioid activity of their own. 



The dominant theory appears to be that codeine is a natural pro-drug for morphine. It is "de-meythlated" in the liver and therefore becomes morphine, which is responsible for its analgesic effects. So I dont believe Codeine's chemical structure "reduces" the activity of codeine, as it is only or primarily its metabolite morphine that is active.
ReplyDeleteI have also read that codeine is not by itself active, and you are right that I was being a little sloppy with the language. What I meant is that a dose of codeine is less active than an equivalent dose or morphine.
DeleteAlthough it does beg the question that if a methyl group added to the oxygen on the #3 carbon deactivates the opiate effect of the molecule, is this also true of hydrocodone and oxycodone? Both of these drugs are also demethylated by the same liver cytochromes as those which convert codeine to morphine in vivo.
This level of chemical theory is above me. It is interesting that it all appears to revolve around the morphine molecule, which is the pre-eminent agoniser (a real word?) of the opiate recepters. Im surprised that you didnt include Heroin in your list. As codeine could be described as a natural pro-drug for morphine, heroin is a semi-synthetic, man made pro-drug for morphine. Heroin has been a godsend for the forces of oppression (prohibition, so-called) as it is used as a vehicle for demonising morphine and opressing morphine users. It is rarely explained to people that Heroin is for practical purposes, morphine. It is portrayed as some sort of dangerous, unique drug which can cause precipitous death. This is of course, a blatant lie. It is just a way of administering morphine.
DeleteCodeine is transformed to morphine in the liver by a family enzymes called cytochrome P450, or CYPs ("sip") for short. The same enzyme that demethylates codeine also demethylates other opiates, transforming hydrocodone to hydromorphone for example.
DeleteYou are right that I should have included heroin, I am actually working on a separate post dedicated to heroin alone. The demonization of heroin is so entrenched in people's minds that it is barely even recognized as an opiate. For all practical purposes, heroin is just fast acting morphine. It is ironic that heroin is class A, having allegedly no medical value, while morphine is class B and considered a vital medication.
How is morphinan numbered? What is the proces of numbering the carbons? Is there a particualr functional group that takes priority?
ReplyDeleteThe International Union of Pure and Applied Chemistry (IUPAC) has their guidelines for naming compounds and specifically how to number the carbons. The nitrogen has the highest atomic number so the carbons would be numbered based on the rules for amines.
DeleteHowever for complex molecules sometimes a larger molecule is used as the backbone and the functional groups are then added. I believe in the morphine image the carbons are numbered using the benzylisoquinoline backbone. Morphinan has a phenanthrene backbone. Depending on which backbone is used the numbering might be different. Most of the time I see morphine-type opiates such as I described here numbered like benzylisoquinolines.
Regarding the relationship morphine to maeng da
ReplyDeleteThe 'codones can at least partly be considered prodrugs for the 'morphones, but evidence from patients with inactive/hyperactive CYP2D6 variants suggests there may be multiple active metabolites.
ReplyDeleteTrue, hydrocodone in particular may be a pro-drug for hydromorphone. However oxycodone is not a pro-drug, although it may well be metabolized to oxymorphone the parent drug is active.
DeletePeople's metabolisms are different and since the morphinan-type opioids all share similar structures its not impossible for a single drug to produce multiple metabolites.
The pain specialist Robert Cochran noted that when he had his patients' urine tested (for compliance purposes), after the lab results came back he often would be more confused then before he had the results.
This comment has been removed by a blog administrator.
ReplyDeleteThis comment has been removed by a blog administrator.
ReplyDeleteThis comment has been removed by a blog administrator.
ReplyDeleteThis comment has been removed by the author.
ReplyDeleteThis comment has been removed by the author.
ReplyDeleteI came here to get some insight into how the opiates actually differ from each other on molecular level, however all I got is a feeling that my 4 years of chemistry classes were probably "little" too basic...damn how I wish I paid more attention ! Anyway, it wasn't all for nothing and next time I'm enjoying that horrible 😱 heroin I can at least pretend I know something about opiates since most of the people know fuck all. Thank you More Pheen for keeping the light at the end of the tunnel of human ignorance burning.
ReplyDeletethanks bro
ReplyDeleteSo is morphinan going to be at least somewhat recreational like u47 or not even worth considering?
ReplyDeleteI have been studing morphinans for a bit now :)
ReplyDeleteI personaly like the synthesis from hexenyl ethylamine
and para methoxy phenyl acetic acid (not Dopamans synth he
has it all screwed up as he did not have access to the origonal articles)
something that I have found that is in line with the diagram you have published is the nitrogen being phenethylated to make a more active substance.
now as of yet I have not tried this and I for the life of me can not find any pharma data on the phenethyl morphinan
derivative other to say it has an activity like fentanyl
ie 100 mics.
with the duration of morphinans being in the 12 too 16 hour range I can not understand the reasoning for there being
a: no pharma data on the substance (I may have missed it)
b: not being of use to the medical world when n methyl morphinan is.
the only idea I can come up with is that it has some reaction
with the histamine system that is not safe or nice for the user.
the other thing I found of interest in the publications I have read is that the salt of the n phenethyl morphinans makes a difference to activity.
HBr salts are the highly active ones were as HCl salts seem to have the same activity as the n methyl analog.
all very weird and not making any sence to me but something to ponder.
this all leads me to be to scared to try and test the n phenethyl analog.
pity as I think it would be a world smasher if it was what it might be...
sorry no refs in an open post like this :)
peace and thanks for the pic it was as I thought.
This comment has been removed by a blog administrator.
ReplyDeleteI read your post and it is really good , keep writing more
ReplyDeleteBuy Weed Seeds Online
Buy A-796,260 Online
Buy Phenethylamine Psychedelics online
Buy DMT Dimethyltryptamine Online
Buy 5-methoxy DALT online
Cannabis Oil For Sale
Buy enzodiazepines online you can as well text +1(646)883-3072 or email:primepharma0@gmail.com for more details.
I started on COPD Herbal treatment from Ultimate Life Clinic, the treatment worked incredibly for my lungs condition. I used the herbal treatment for almost 4 months, it reversed my COPD. My severe shortness of breath, dry cough, chest tightness gradually disappeared. Reach Ultimate Life Clinic via their website www.ultimatelifeclinic.com. I can breath much better and It feels comfortable!
ReplyDelete